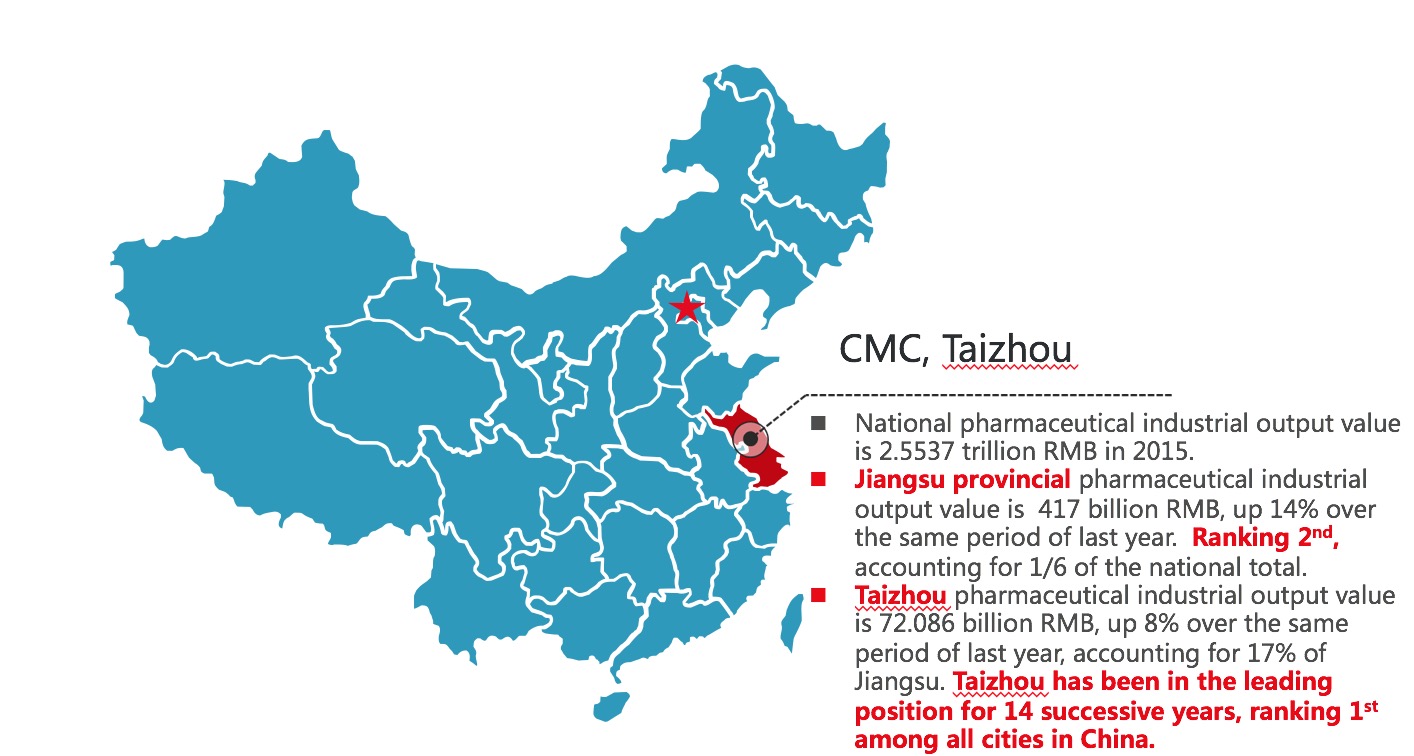
Industrial background
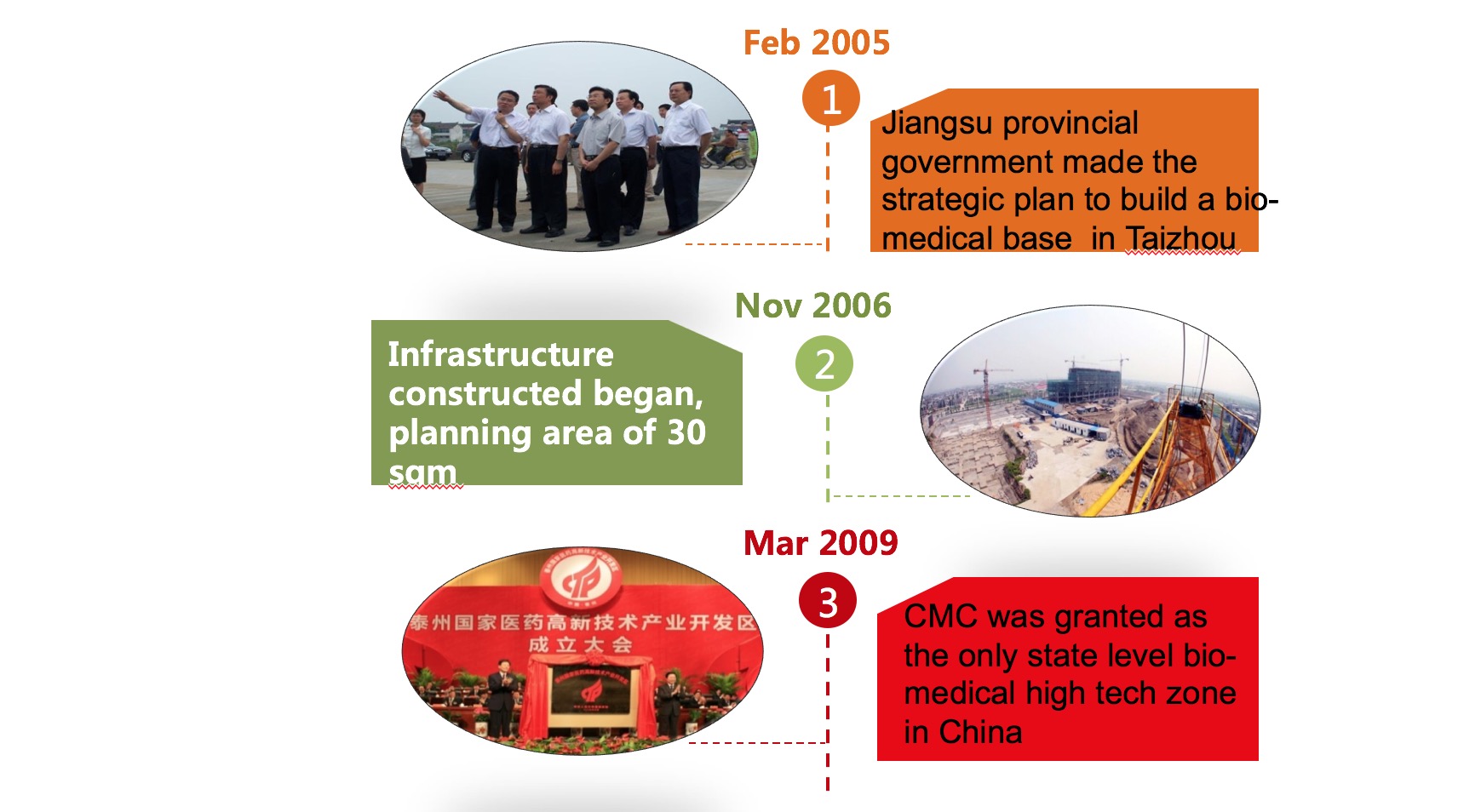
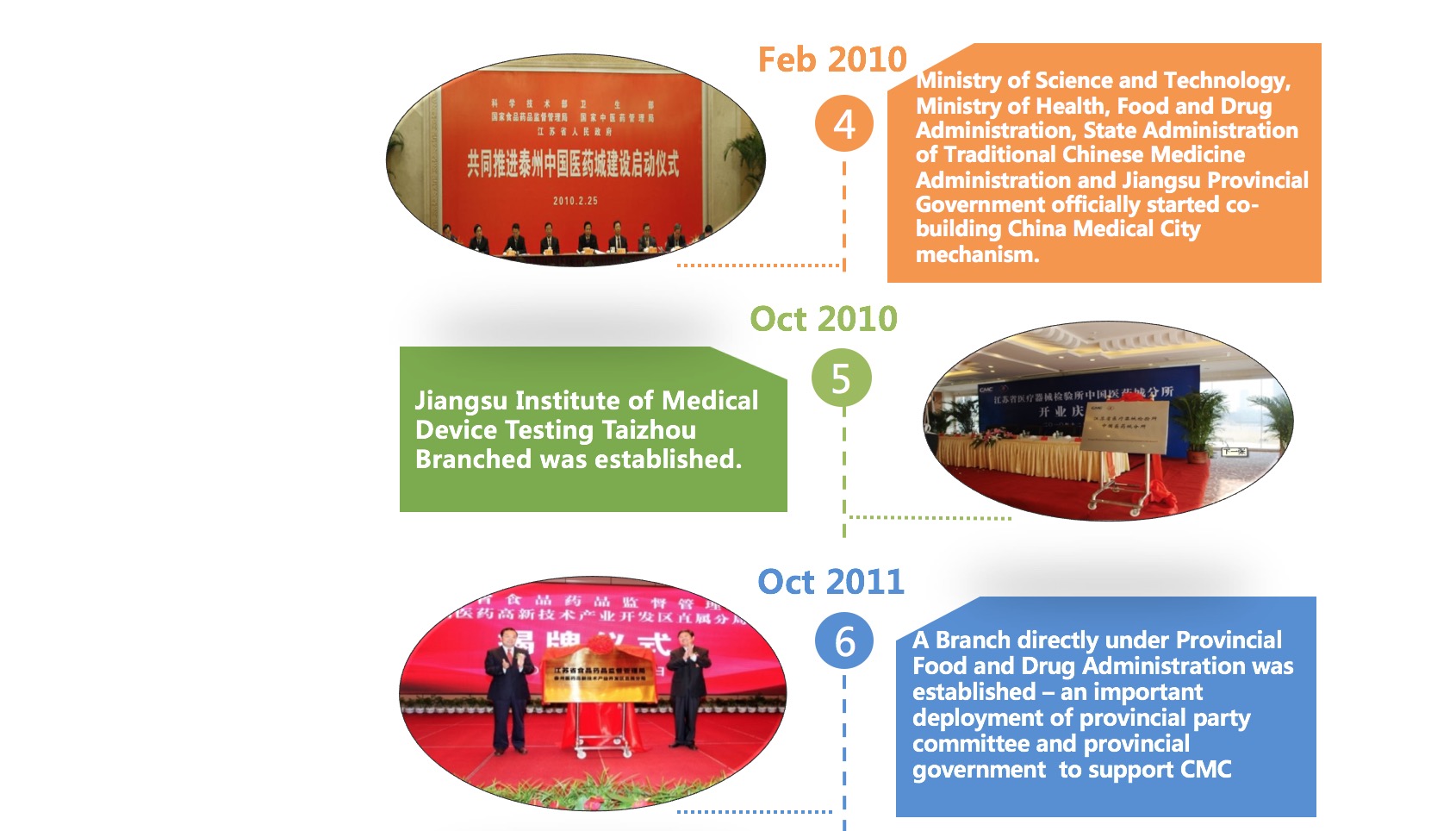
Development History
Industry Cluster
132 drug R&D and manufacturing enterprises
- 30 enterprises have obtained production certificates, 37 production lines of 14 enterprises have passed GMP certification.
- 25 enterprises have obtained drug distribution certificates, 25 have passed new GSP certification.
- Owning a variety of 560 drugs, among which 56 are Class 1 new drugs.
- 95 have obtained approved documents for production, 131 for clinical application and over 60 varieties are under examination and approval.
262 medical devices R&D and manufacturing enterprises
- 80 enterprises have obtained production certificates for medical equipment.
- Currently, 1214 varieties are under R&D and application. 602 have been registered or obtained filling certificates (269 Class 2, 3 products); 612 are under review and evaluation.
20 health-care food/ cosmetics R&D and manufacturing enterprises
- 10 enterprises have obtained food production certificates (4 are health-care food manufacturers)
- 3 enterprises have obtained cosmetics production certificates.
- 25 types of health-care food and 94 kinds of cosmetics have obtained certificates.










Service System
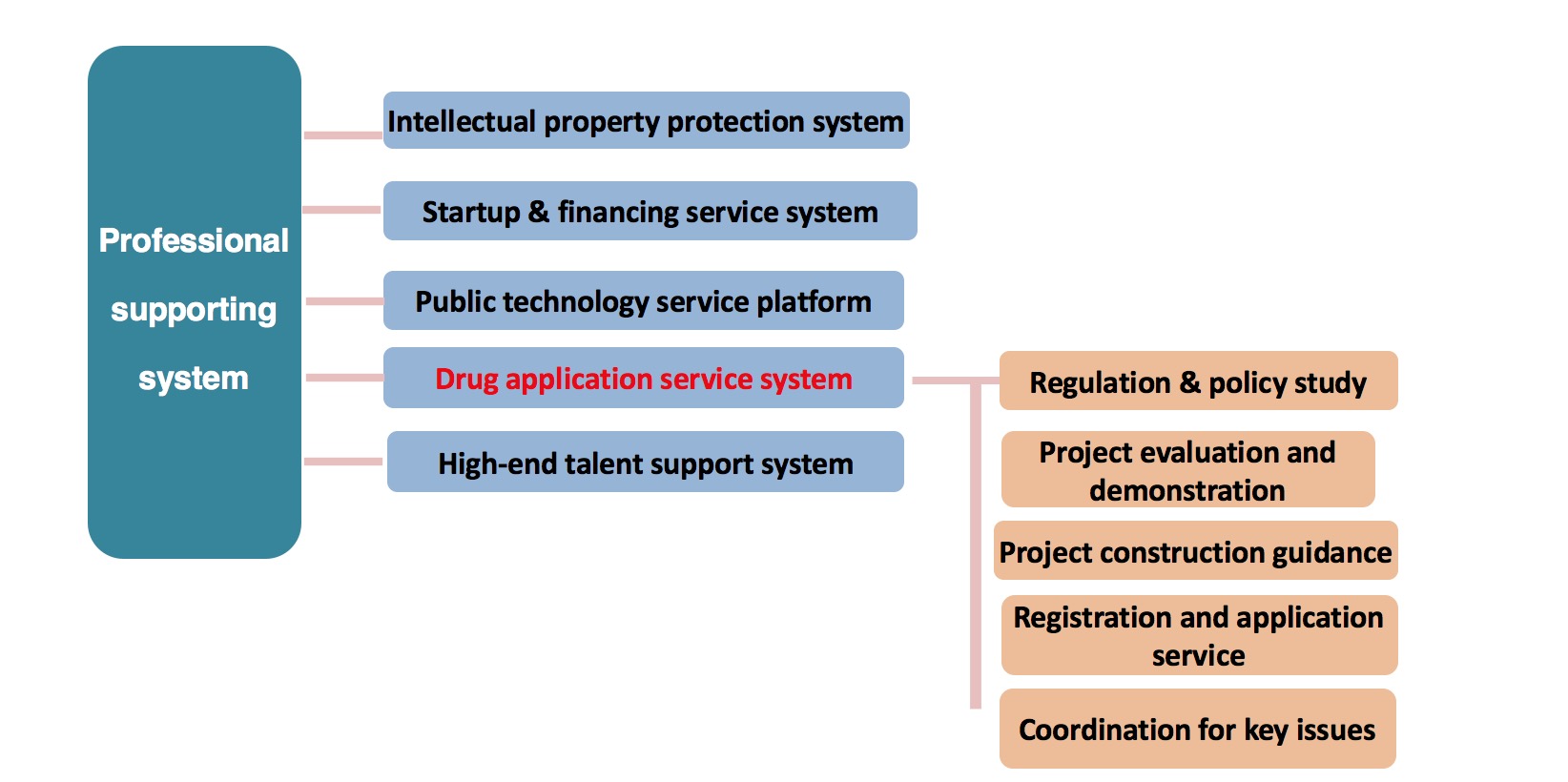
Function of administrative licensing
The setup of direct branch has achieved a point-to-point reporting service system for the enterprises in China Medical City, becoming an important supporting force to promote the development of China Medical City and adding new impetus to the construction of “a medical city combining production and residence”.
Responsibility of Jiangsu Food and Drug Administration, CMC Branch
- Under the authorization of the provincial administration, bear the relevant licensing duties of registration, production and operation of drugs, medical devices, health food and cosmetics within the scope of China Medical City.
- Guide the daily supervision and management work of the “three products and one device” production and operation within the scope of China Medical City.
- Provide relevant services for the industry development of China Medical City.


Administrative examination & approval work carried out by Direct Branch
| Administrative examination & approval matter | Mode |
|---|---|
| Review and issuance of drug production certificates | Full |
| Review and issuance of certificates for medical devices manufacturing enterprises (Class 2, 3) | Full |
| Review and issuance of certificates for drug distribution enterprises | Full |
| Review and issuance of business certificates for medical devices (Class 2, 3) | Full |
|
Drug registration 1: Registration and application of drug clinical research 2: Registration and application of new drug production 3: Registration of existing state standard drugs | Full |
Technical appraisal: participate in appraisal of variety transfer technology materials of No.38 Document; inspect and review the examination and registration system of Class 2 medical
Work Measures
Early-stage guidance, full service
- Jointly study and make project landing plan;
- Plant drawings demonstration, document preview;
- Expert onsite guidance, simulated inspection;
- Regulation study, training & publicity.
Communication mechanism
- National ministers communication mechanism with posts (Ministry of Science and Technology, CFDA, State Administration of Traditional Chinese Medicine);
- Study and communication mechanism with posts reserved (Ministry of Science and Technology, CFDA, CDE, National Institutes for Food and Drug Control, etc.);
- CDE examination and communication system (major and special varieties, innovative devices, clinical value assessment).
Optimize Procedure, reduce time limit
- Principle: maintain standards and procedures, improve speed;
- Expert preview, simulated inspection;
- Drug and medical devices review participation and onsite inspection;
- Simplify steps, combine inspections.
System innovation, pilot study
- Drug registration specialist system;
- Pharmaceutical study trend filing & management system and informatization system;
- Promote informatized examination & approval system;
- Drug manufacturing and operation quality regular meeting system;
- Project study, MAH and other pilot studies.
Early-stage guidance, full service


Large social good“ Generic Drug Consistency Evaluation” seminar

MAH investigation and survey meeting

Drug registration specialist training class

GMP training class
Branch drug manufacturing and operation certificate handling time limit
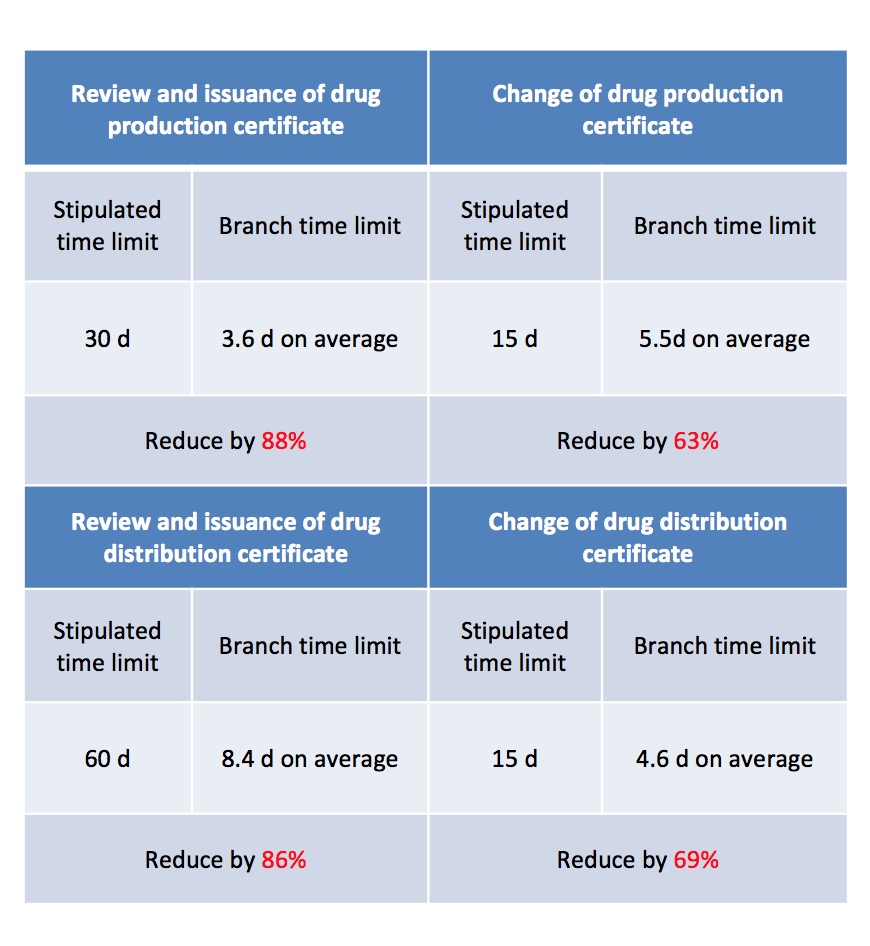
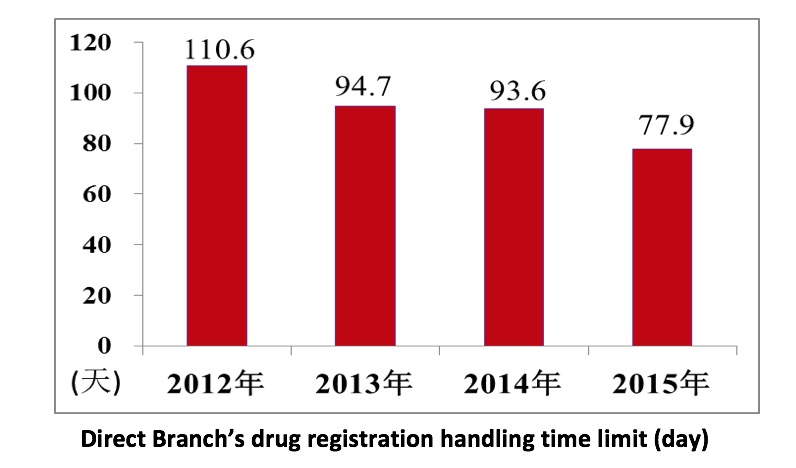
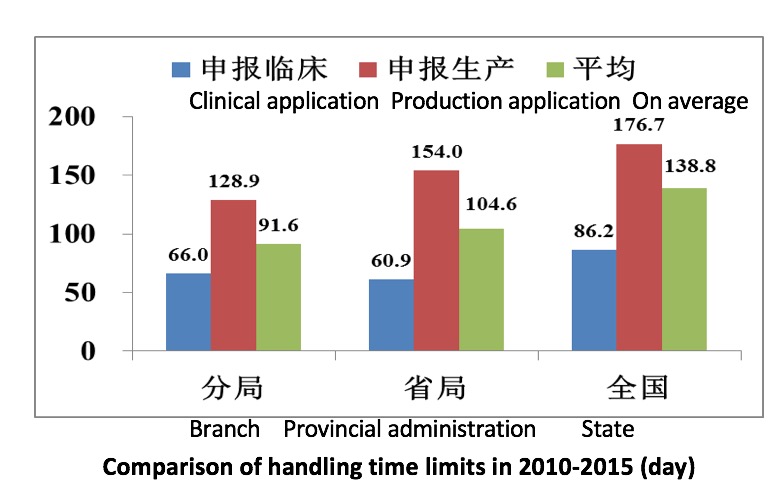
System innovation, pilot study
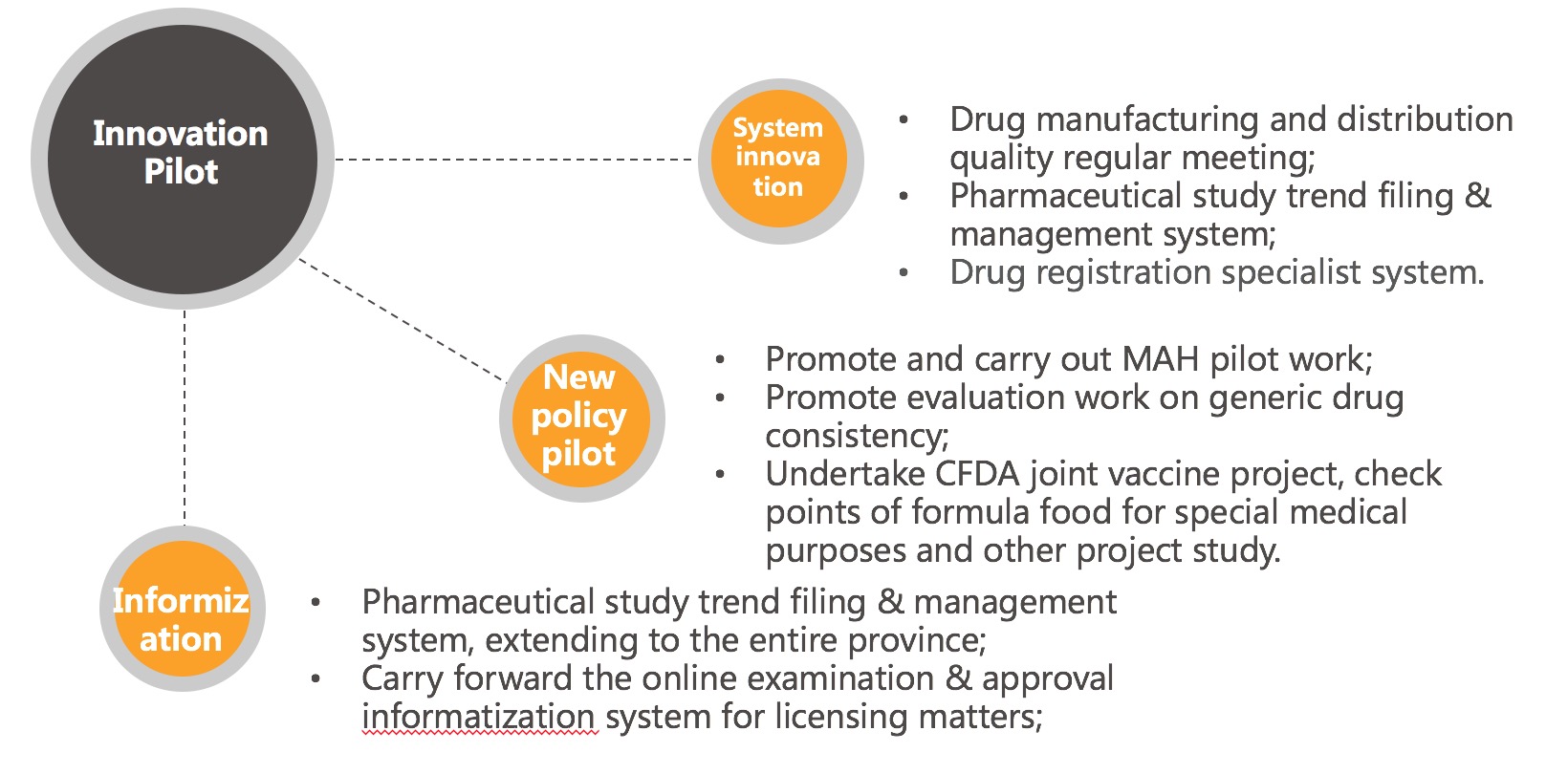
Deepen ministry-province joint construction mechanism
Ministry-Province joint construction & meeting system
- Annual minister-level joint meeting; quarterly contact person meeting.
- Carefully study the needs of industrial development of the zone, put forward reasonable requirements according to national policies and directions.
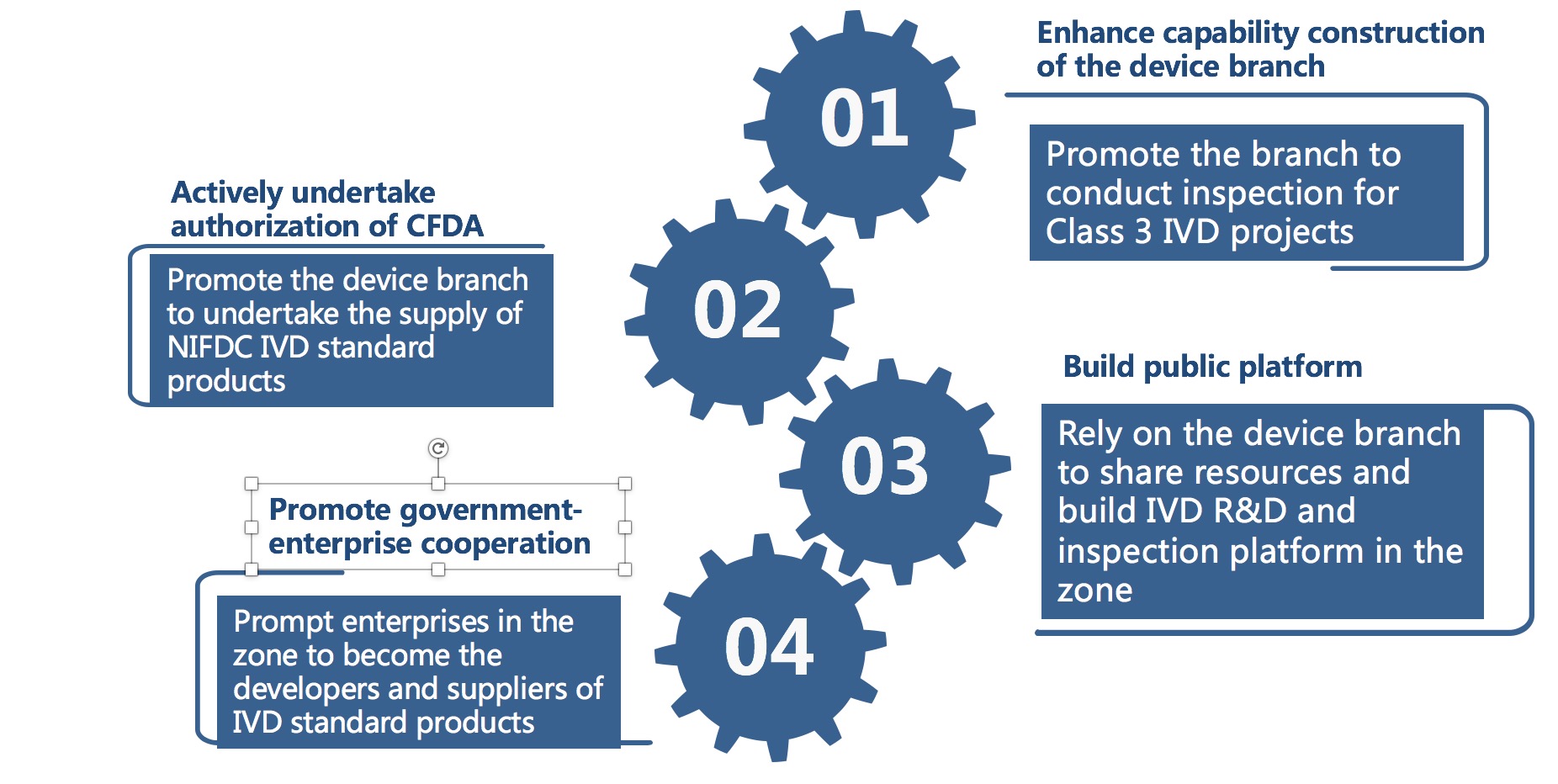
Undertake the business authorization of CFDA
- Promote the cooperation concerning the development, preparation and supply of in vitro diagnostic reagent standard products between the device branch and National Institutes for Food and Drug Control;
- In 2016, the provincial device branch passed the CMA & CNAS two-in-one onsite examination, and has the capability of detecting 72 products and parameters covering 7 categories including biochemistry, immunity and nucleic acid. Last year, it undertook nearly half of the IVD inspection batches and amount in the province.
- Promote the device branch to carry out Class 3 IVD inspection project.
Communication with posts reserved
- National ministers communication mechanism with posts (Ministry of Science and Technology, CFDA, State Administration of Traditional Chinese Medicine);
- Study and communication mechanism with posts reserved (Ministry of Science and Technology, CFDA, CDE, National Institutes for Food and Drug Control, etc.)
Regular communication to facilitate examination
- Conduct special coordination for varieties such as hepatitis A inactivated vaccine and Nafamostat Mesilate, and expedite review.
- Coordinate and promote the application of innovative medical device.
- Promote the legislation of formula food for special medical purposes.
Commissioning of AstraZeneca Taizhou Supplying Site
- Lasted only 31 months from groundbreaking to passing the GMP certification.
- Taizhou plant received President Award of AstraZeneca.
- Awarded by ISPE in 2015.



- Jointly study on variety transfer plans;
- Participate in the drawings design and guide on application documents of AZ Taizhou plant;
- Arrange expert onsite guidance and simulated inspection;
- On the premise of compliance, reduce the time of variety
- Transfer as much as possible.
- Combing inspections of certificate change and review & issuance;
- Combining variety transfer validation approval and GMP certification approval.
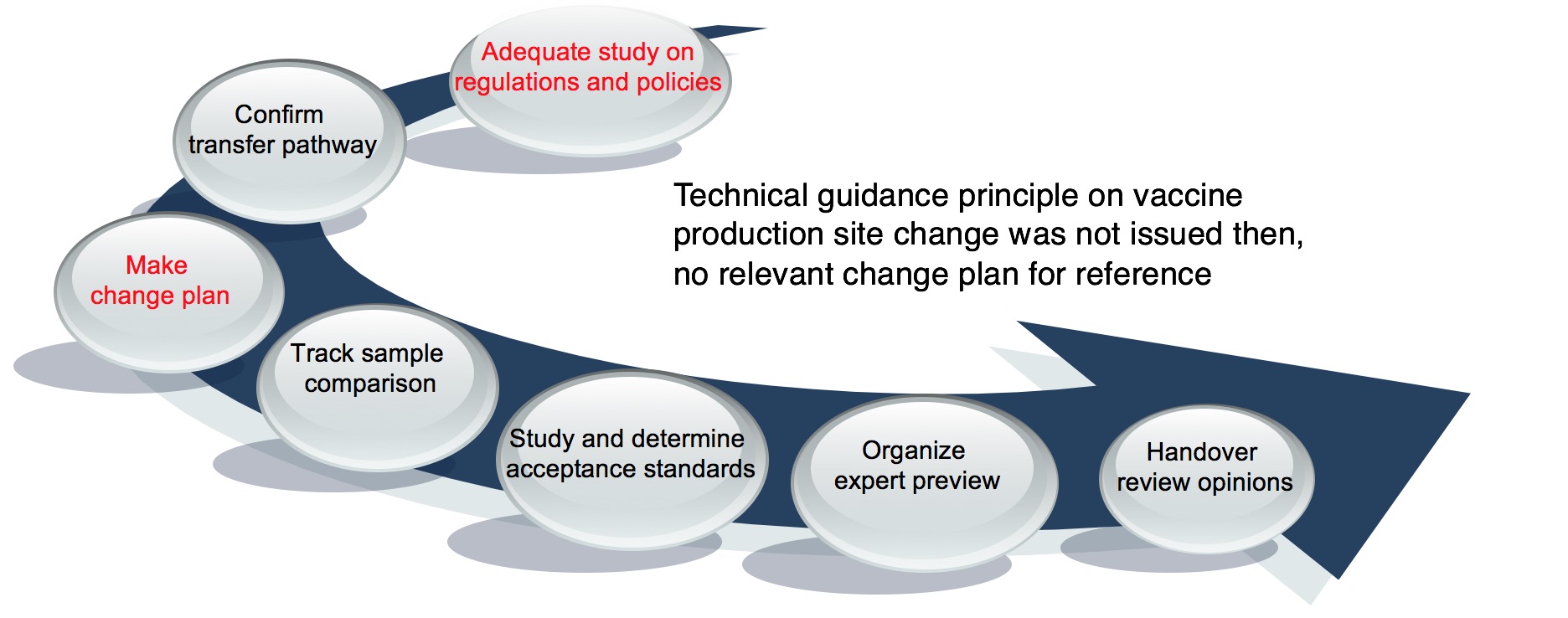
Landing and industrialization of Convac Bio HAV
Direct Branch actively got involved, coordinated each regulatory resource, guided and supported from beginning to end, and the product transfer plan was highly recognized. It took 10 months to obtain the production approval documents.
IVD standard product preparation collaboration with NIFDC
Other major results
- Junshi Biosciences PD1 First company approved to do clinical trials
- MicroMedMark nucleic acid case First in the world
- BioPerfectus HPV classification and quantitative system First in China
- Nestle China Promote legislation of formula food for special medical purposes
- Hwaway Medical Platform Third-party logistics platform for drugs

